News
Interpretation of Decree of the General Administration of Customs No.248
4/27/21 GACC
The decree of the General Administration of Customs No.248 (Decree on the issuance of the “Regulations on the Registration and Administration of Overseas Production Enterprises of Imported Food of the People’s Republic of China”) was published on April 12th, 2021, and it will be implemented starting from January 1st 2022…
CSMS #42635055 – FDA Document Submission During COVID-19 Pandemic
05/08/2020 U.S. CBP
– If a request for documents is received from FDA, filers and/or importers are strongly encouraged to submit product labeling along with regularly submitted entry documents (commercial invoice, packing list, way bill, etc.)…
Chinese, U.S. chief trade negotiators hold phone talks
05/08/2020 Source: Xinhua
– Chinese Vice Premier Liu He, a member of the Political Bureau of the Communist Party of China Central Committee and chief of the Chinese side of the China-U.S. comprehensive economic dialogue, held a phone conversation with U.S. Trade Representative Robert Lighthizer and Treasury Secretary Steven Mnuchin Friday morning.
The two sides agreed that…
Business is booming for these 14 companies during the coronavirus pandemic
05/07/2020 New York (CNN Business)
– The coronavirus pandemic has been, to say the least, grim for business. Widespread layoffs and furloughs have prompted about 21% of the US labor force to file for unemployment benefits since mid-March, and economists say the United States is likely already in a recession. And even as states begin to reopen, many of the jobs that have been lost may never come back.
China Makes Biggest U.S. Pork Order of 2020 as Virus Hits Plants
05/07/2020 Bloomberg
– China is ramping up imports of U.S. pork, going against the trend as America’s meat exports have slowed overall with sick employees limiting production at meatpacking plants…
USDA To Implement President Trump’s Executive Order On Meat and Poultry Processors
04/28/2020 USDA
– U.S. Secretary of Agriculture Sonny Perdue released the following statement after President Donald J. Trump signed an Executive Order to keep meat and poultry processing facilities open during the COVID-19 national emergency…
The 127th Canton Fair to Be Held Online on June 15-24
04/17/2020 MINISTRY OF COMMERCE, PRC
– The 127th China Import and Export Fair (Canton Fair) will be held online on June 15-24 for 10 days. Holding the Canton Fair online is an innovative measure to actively deal with the impact of COVID-19 and to strive to stabilize the fundamentals of foreign trade and foreign investment…
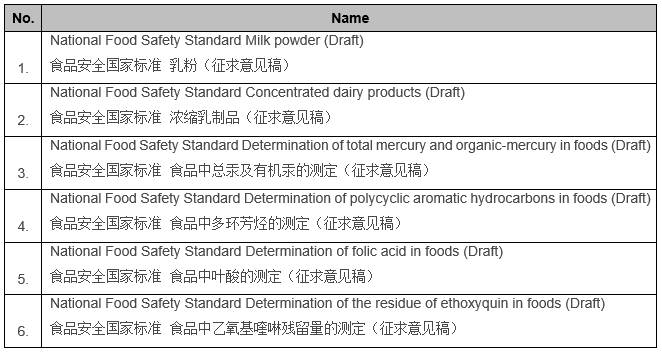
6 Chinese National Food Safety Standards are open for public opinions
04/08/2020 NATIONAL HEALTH COMMISSION, PRC
– The Secretariat of the National Food Safety Standard Review Committee of the National Health Commission publicly solicited opinions on 6 national food safety standards (draft for comment).
The deadline for feedback is June 3, 2020.
http://www.nhc.gov.cn/sps/s3593/202004/58d4c36cac634d16bb1a0ee0dc40fb19.shtml
03/31/2020 GACC
– Beginning April 1st, enterprises exporting new Coronavirus detection reagents, medical masks, medical protective clothing, ventilators, and infrared thermometers must submit paper or electronic statement to the Customs, committing the export product has obtained China’s medical device product registration certificate, and meet the importing country (region) quality standards…
USDA and USTR Announce Continued Progress on Implementation of U.S.-China Phase One Agreement
03/24/2020 USDA
– The U.S. Department of Agriculture (USDA) and the Office of the U.S. Trade Representative (USTR) today announced continued progress in the implementation of the agriculture-related provisions of the U.S.-China Phase One Economic and Trade Agreement…
510(k) Third Party Review Program – Guidance for Industry, Food and Drug Administration Staff, and Third Party Review Organizations External Link Disclaimer
03/12/2020 FDA
– The 510(k) Third Party (3P510k) Review Program (formally known as the Accredited Persons (AP) Program) is authorized under section 523 of the Federal Food, Drug, and Cosmetic (FD&C) Act. Under this authority, FDA recognizes third parties to review premarket notification (510(k)) submissions and recommend the initial classification of certain devices…

